Table of Contents
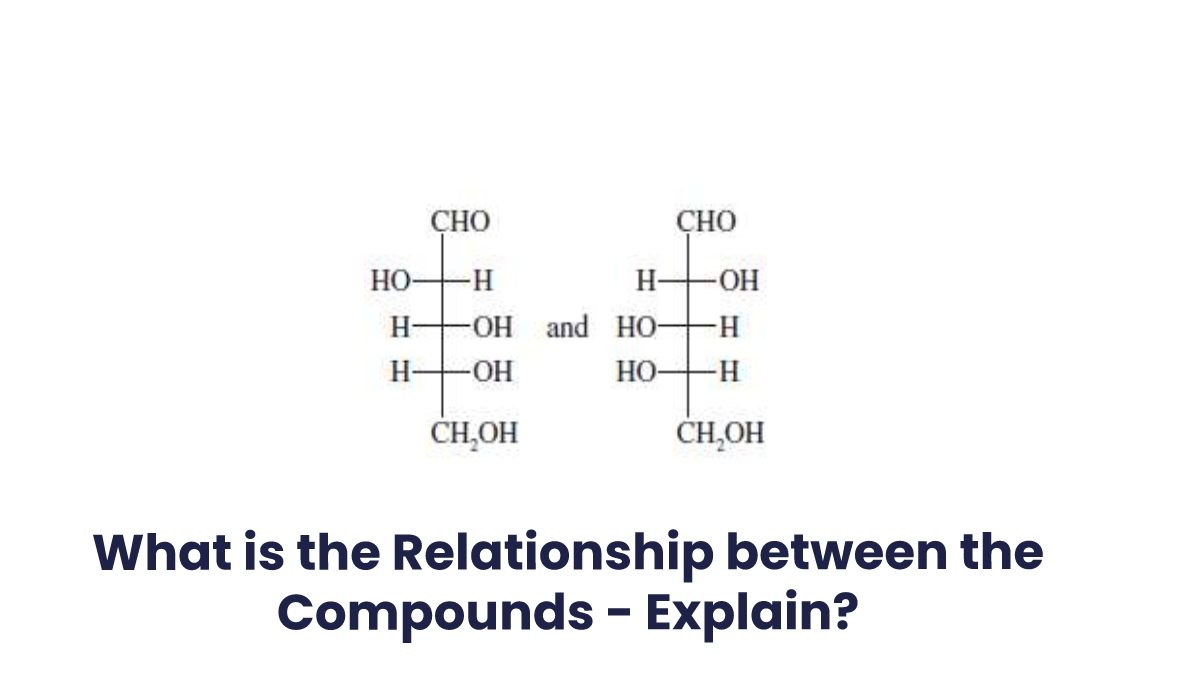
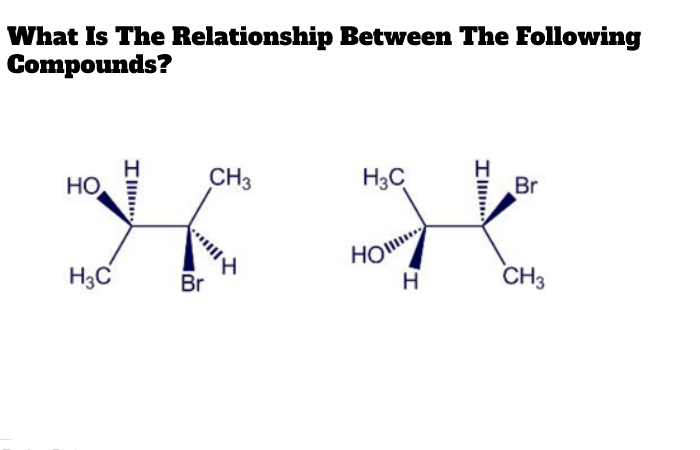
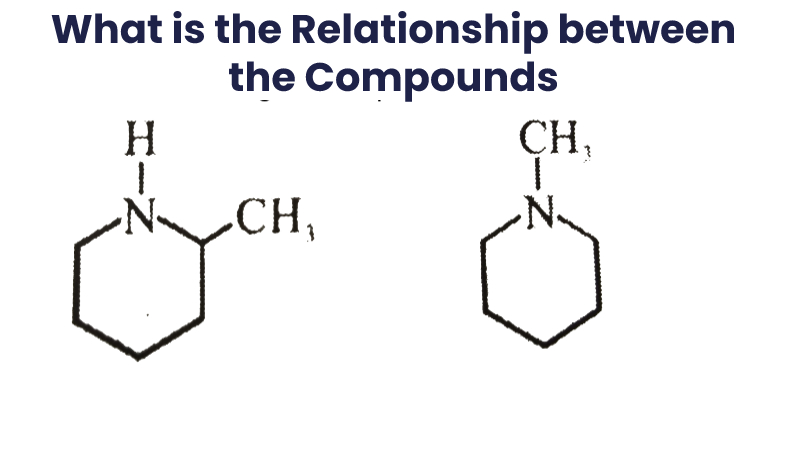
What Is The Relationship Between The Following Compounds?
Constitutional Isomers Enantiomers Diastereomers
Enantiomers and diastereomers are the only two stereochemical relationships that you can have between any two molecules. The stereoisomers are any two molecules that fulfill the following two requirements:
- Both molecules must have the same molecular formula, and
- Both molecules must have the same atom connectivity.
So, What’s the difference then? The molecules are stereoisomers if they have a different #D shape and they are not superimposable in space.
A molecule is a set of two or else more atoms chemically bonded. It includes the carbohydrates you eat, the water you drink, and the oxygen you breathe. Specific molecules are also compounds. A compound is a group of two or more different atoms chemically bonded together.
A molecule remains formed when two or more atoms of the same element chemically bond, while a compound remains defined as the chemical bond between atoms of different factors. Learn about the relationship between parts, molecules and compounds.
What are Elements?
The other day, I was at the jewellery store looking at all the different bangles, earrings, necklaces, and rings. Some jewellery pieces were simple: a gold ring, a platinum bracelet, a pair of silver cufflinks. Others were more complex: ruby and emerald necklaces, diamond tennis bracelets, and jade rings. It got me thinking about the periodic table because I know I can find gold, silver, and platinum in it. But no substance how hard I try, I won’t find ruby, emerald, or jade there.
What struck me is that I also find things like helium, oxygen, sodium, and lead in the periodic table. These do not seem to do with gold, silver, and platinum, but they have something significant in common. They are all elements! An element is a material prepared of only one type of atom. It is why those beautiful gemstones in jewellery have no place on the periodic table: they remain made up of multiple elements.
Molecules are Groups of Atoms
While many things in nature come in their pure elemental form, there are, of course, many other things that do not. Instead, they are combinations of elements. When elements combine, they form molecules. A molecule is a set of two or additional atoms chemically bonded together.
Molecules can be simple, or they can be pretty complex. For example, some simple molecules include the oxygen you breathe.
Which remain made up of two oxygen atoms, the salt you put on your food, which comprises one sodium atom and one chlorine atom, or the water you drink, which remain prepared up and about of two hydrogen atoms and one oxygen atom.
A more complex molecule would be something like glucose, a sugar with numerous carbon, hydrogen, and oxygen atoms bonded together.
What is not a molecule, then? Well, this would be an individual atom of any element. If it’s not paired with at least one other particle, it’s not a molecule. Molecules follow the buddy system!
Compounds are different Elements
Molecules are groups of two or extra atoms, but there is also a particular subgroup of molecules. These are compounds, and a combination is a group of two or more different atoms chemically bonded together.
The key here is that the compounds remain made of at least two other elements. So this would include your water since it remains made of hydrogen and oxygen, as well as your salt since it’s made of sodium and chlorine.
However, the oxygen you breathe is not a compound because it only contains oxygen. But it is still a molecule because it is a combination of at least two atoms. It can be unclear because all compounds are molecules, but not all molecules are compounds. Let’s look at some more examples to see how this works.
FAQS
What Is The Relationship Between The Following Compounds?
A Grignard reagent reacts with water to give:
Solution: Grignard reagent reacts with water (acidic H) to give alkane.
Here Grignard acts as a base.
CH3CH2MgBr+H2O→CH3CH3+Mg(OH)Br
Assertion (A): Ethyl chloride gives ethyl alcohol when treated with Ag2O in boiling water.
Reason (R): On boiling, the vapour pressure of water increases.
Solution: Alc. KOH is a dehydrohalogenation agent and it forms alkene.
It’s a basic preparation method for alkyl alcohol, in this process alkyl halide converts into alcohol in presence of aq. KOH/NaOH.